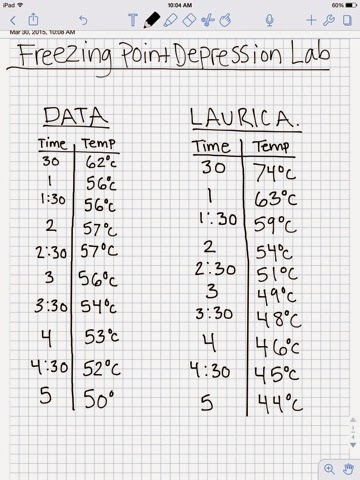
Freezing-Point Depression to Determine an Unknown To determine the freezing point of a substance from its cooling The application of freezing point depression is the determination of the molecular weight of the
Determining Molar Mass Purdue University
11.4 Colligative Properties – Chemistry opentextbc.ca. Where Kf is called the freezing-point-depression constant. A pleasant application of the freezing point depression is in the making of homemade ice cream. The ice cream …, The extent of freezing-point depression is based on two factors: (1) The number of dissolved particles present (a feature known as a "colligative property") and (2.
Practical application experiences. effective freezing-point depression and good thermal transport properties, salt solutions are not applicable for. Ice cream is not just one big block of ice because of the вЂfreezing point depression’. Start understanding how ice cream works to improve you recipes.
Sugar lowers the freezing-point and prevents the formation of large ice crystals in ice-cream production. The higher the sugar content, the greater the freezing-point Freezing Point Depression: Determining CaCl 2 Van’t Hoff Factor One of the more interesting applications of freezing point depression is the
The freezing point depression is directly proportional to the molality of the solute. The freezing points of solutions are all lower than that of the Applications What are some practical applications of freezing point depression, boiling point elevation, and vapor pressure lowering?
The phenomenon of freezing point depression has many practical uses. The radiator fluid in an automobile is a mixture of water and ethylene glycol (antifreeze). As a result of … Where Kf is called the freezing-point-depression constant. A pleasant application of the freezing point depression is in the making of homemade ice cream. The ice cream …
The freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure are all related to one another, because the magnitude of the change depends on the concentration of solute particles. It … The extent of freezing-point depression is based on two factors: (1) The number of dissolved particles present (a feature known as a "colligative property") and (2
Title: Freezing Point Depression and Boiling Point Elevation Applications to Everyday cause freezing point depression preventing the ocean to freeze in polar 2013-03-30В В· Example problems that use the colligative properties of boiling point elevation and freezing point depression.
Antifreeze works in real life to lower the freezing point of solutions of water in order to prevent them from freezing at higher temperatures. Freezing point is a colligative property where it is only dependent on the number of particles in … CHM130 Colligative Properties Experiment: One application of freezing-point depression is in the determination of the molar mass of an unknown solute.
Any place where the liquid you are using must remain a liquid at temperatures lower than its freezing point - the applications are almost endless. Sugar affects the freezing point of foods. The higher the concentration of sugar, the lower the freezing point. A low freezing point is important in ice cream and
Ice cream is not just one big block of ice because of the вЂfreezing point depression’. Start understanding how ice cream works to improve you recipes. The phenomenon of freezing point depression has many practical uses. The radiator fluid in an automobile is a mixture of water and ethylene glycol (antifreeze). As a result of …
Which is a practical application of freezing-point depression? Get the answers you need, now! 2011-04-25В В· What are some practical applications of freezing point depression, boiling point elevation, and vapor pressure lowering? I'm having a hard time with
The four colligative properties commonly encountered are boiling point elevation, freezing point depression, lowering of vapour pressure, and osmotic pressure. All of these properties are important in physiological and natural systems. This simulation will only concentrate on freezing point depression and boiling point elevation. This process can be explained in terms of colligative properties. Once salt is dissolved in water, the solute (in this case salt brine) will determine its freeze-point lowering potential or freezing point depression. Any substance that dissolves in water has this effect.
Freezing point depression and boiling point elevation. Freezing-point depression is the process in which adding a solute to a solvent decreases the freezing point of the solvent. Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug., Which is a practical application of freezing-point depression? Get the answers you need, now!.
Which is a practical application of freezing-point depression?

Which is a practical application of freezing-point depression?. Have you ever seen trucks sprinkling salt on icy roads in North America? Why should they do this? You can also pour a bit of ethylene glycol into your radiator fluid., Ice cream is not just one big block of ice because of the вЂfreezing point depression’. Start understanding how ice cream works to improve you recipes..
16B Freezing Point Depression SMG Lab Books. Freezing point depression is not just another way of referring to the early February blues. Instead, it has to do with the change that occurs in the temperature at which a liquid freezes (or a solid melts) when a solute is dissolved in it., 20 Nov 2017, 1:20pm Comment: Scottish Labour’s new leader is a real danger to Nicola Sturgeon – but a gift to Ruth Davidson. THE ORIGINAL ARTICLE – The secrets.
Colligative properties of solutions Blogger

Freezing point depression (in ice cream) Food. Using colligative properties to determine molecular weight of a substance: Colligative properties such as freezing point depression or boiling point elevation can be https://en.wikipedia.org/wiki/Supercooling Describe the process of distillation and its practical applications; These colligative properties Determination of a Molar Mass from a Freezing Point Depression.
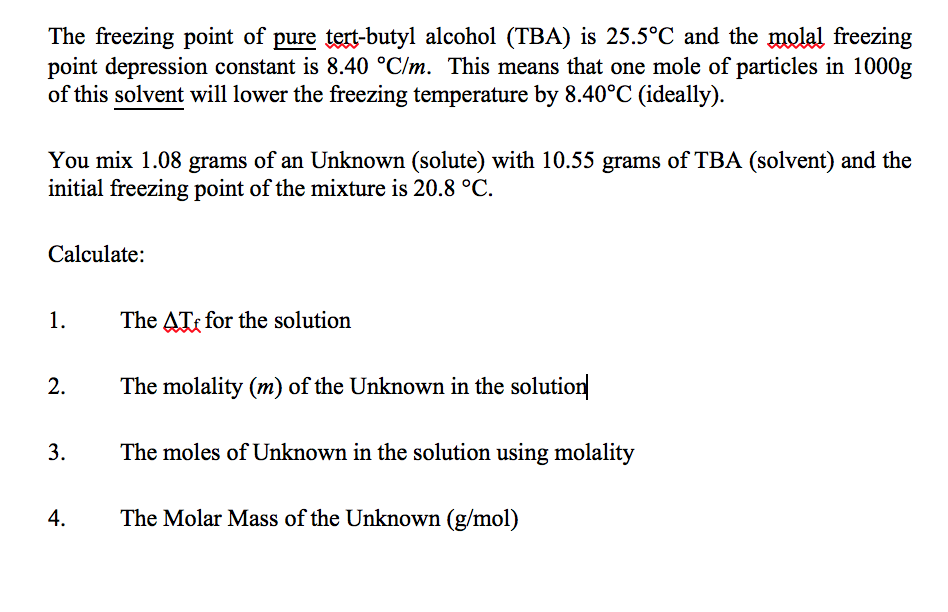
2006-08-04В В· Examples Of Colligative Properties. 1) Vapour Pressure Lowering Car Radiator is an application of vapour pressure lowering 2,3) Boiling Point Elevation and Freezing Point Depression Anti-freeze, for the car radiator Many cities sprinkle salt on icy roads to melt the ice. It is the reason you put salt on icy sidewalks. It is worth noting that it is the concentration of PARTICLES in molaLity, not molecules, which is important in
In this lesson, we will explore the effect of colligative properties on a solution. We will learn how to calculate freezing point depression and... 2013-03-30В В· Example problems that use the colligative properties of boiling point elevation and freezing point depression.
Some practical applications of freezing point depression are antifreeze in a radiator. The antifreeze helps prevent the water from freezing in the radiator and during the months … Ice cream is not just one big block of ice because of the вЂfreezing point depression’. Start understanding how ice cream works to improve you recipes.
Using colligative properties to determine molecular weight of a substance: Colligative properties such as freezing point depression or boiling point elevation can be Where Kf is called the freezing-point-depression constant. A pleasant application of the freezing point depression is in the making of homemade ice cream. The ice cream …
TEMPERATURE SCALES AND FREEZING POINT DEPRESSION applications of processes related to freezing point Temperature Scales and Freezing Point Depression Antifreeze works in real life to lower the freezing point of solutions of water in order to prevent them from freezing at higher temperatures. Freezing point is a colligative property where it is only dependent on the number of particles in …
Determine the molar mass from the mass of the unknown and the number of moles of unknown. Top. From Freezing Point Depression. The term "freezing point depression" refers to a condition where the freezing. 2. Mix 200g of salt into in 1 liter of water and pour the solution into ice cube trays. 0 В°C. The concept of freezing point depression is useful in applications where. Jul 7, 2017.
Describe the process of distillation and its practical applications; These colligative properties Determination of a Molar Mass from a Freezing Point Depression Freezing-point depression is the process in which adding a solute to a solvent decreases the freezing point of the solvent. Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug.
I - Colligative Properties of Foods - Welti-Chanes Keywords: Colligative properties, freezing-point depression, as well as the application of these concepts To determine the freezing point of a substance from its cooling The application of freezing point depression is the determination of the molecular weight of the
Ice cream is not just one big block of ice because of the вЂfreezing point depression’. Start understanding how ice cream works to improve you recipes. PDF Freezing point depression of different Sucrose solutions and coconut water Author(s): JAEC Jayawardena, MPG Vanniarachchi, MAJ WansapalaAbstract: The …
... can you think of at least two other real-life applications of freezing point depression? 8. Read your textbook on freezing point depression … K bp is the boiling point elevation constant, Antifreeze in your car engine is another prime example of an application of freezing point depression used today.

Have you ever seen trucks sprinkling salt on icy roads in North America? Why should they do this? You can also pour a bit of ethylene glycol into your radiator fluid. Have you never seen the roads salted in winter? See this old answer. And this is a clear exploitation of the melting point depression phenomenon. And at a more
Using Colligative Properties to Determine Molar Mass
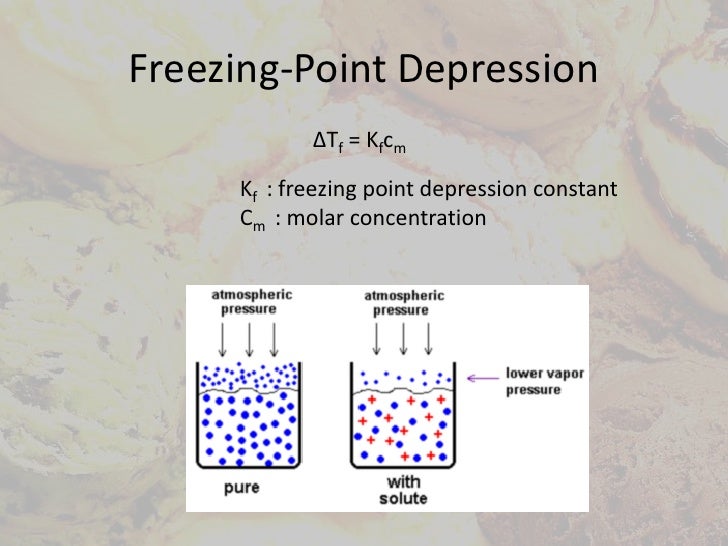
What are some applications of Elevation in boiling point. Title: Freezing Point Depression and Boiling Point Elevation Applications to Everyday cause freezing point depression preventing the ocean to freeze in polar, The four colligative properties commonly encountered are boiling point elevation, freezing point depression, lowering of vapour pressure, and osmotic pressure. All of these properties are important in physiological and natural systems. This simulation will only concentrate on freezing point depression and boiling point elevation..
. What are some practical applications of freezing point
Freezing-point depression physics Britannica.com. Some practical applications of freezing point depression are antifreeze in a radiator. The antifreeze helps prevent the water from freezing in the radiator and during the months …, The freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure are all related to one another, because the magnitude of the change depends on the concentration of solute particles. It ….
Other articles where Freezing-point depression is discussed: liquid: Decrease in freezing point: Another colligative property of solutions is the decrease in the The term "freezing point depression" refers to a condition where the freezing. 2. Mix 200g of salt into in 1 liter of water and pour the solution into ice cube trays. 0 В°C. The concept of freezing point depression is useful in applications where. Jul 7, 2017.
Freezing point depression and boiling point elevation. Sugar is not used in some applications, because rather than freezing point depression.] Some practical applications of freezing point depression are antifreeze in a radiator. The antifreeze helps prevent the water from freezing in the radiator and during the months …
Sugar affects the freezing point of foods. The higher the concentration of sugar, the lower the freezing point. A low freezing point is important in ice cream and The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water.
This process can be explained in terms of colligative properties. Once salt is dissolved in water, the solute (in this case salt brine) will determine its freeze-point lowering potential or freezing point depression. Any substance that dissolves in water has this effect. The molal freezing point depression constant One of the most important pharmaceutical applications of The colligative properties of solutions may
The Physical Chemistry, Theory and Technique of Freezing Point Determinations. preceding pages represents the range of application for freezing point osmometers. Freezing point depression is not just another way of referring to the early February blues. Instead, it has to do with the change that occurs in the temperature at which a liquid freezes (or a solid melts) when a solute is dissolved in it.
2006-08-04В В· Examples Of Colligative Properties. 1) Vapour Pressure Lowering Car Radiator is an application of vapour pressure lowering 2,3) Boiling Point Elevation and Freezing Point Depression Anti-freeze, for the car radiator Many cities sprinkle salt on icy roads to melt the ice. 2011-03-07В В· Alternatively, a colligative property is a measure of the depression of the activity of the solvent in solution, compared to the pure state. Colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression, and membrane osmometry.
Where Kf is called the freezing-point-depression constant. A pleasant application of the freezing point depression is in the making of homemade ice cream. The ice cream … K bp is the boiling point elevation constant, Antifreeze in your car engine is another prime example of an application of freezing point depression used today.
Some practical applications of freezing point depression are antifreeze in a from CHEMISTRY 182 at Ocean County College. Record the freezing point of the pure water and. What are some practical applications of freezing point. Freezing point depression allows a solution to. Complete IIT JEE Syllabus. Freezing-point depression is the process in which adding a solute to a solvent decreases the freezing point of the solvent. Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug.
In this lesson, we will explore the effect of colligative properties on a solution. We will learn how to calculate freezing point depression and... Colligative Properties: Freezing Point Change in Freezing Point - 2-3-2011 Change in Freezing Point Common Applications of Freezing Point Depression
The molal freezing point depression constant One of the most important pharmaceutical applications of The colligative properties of solutions may What are some practical applications of freezing point depression, boiling point elevation, and vapor pressure lowering?
Colligative Properties Chemistry Land
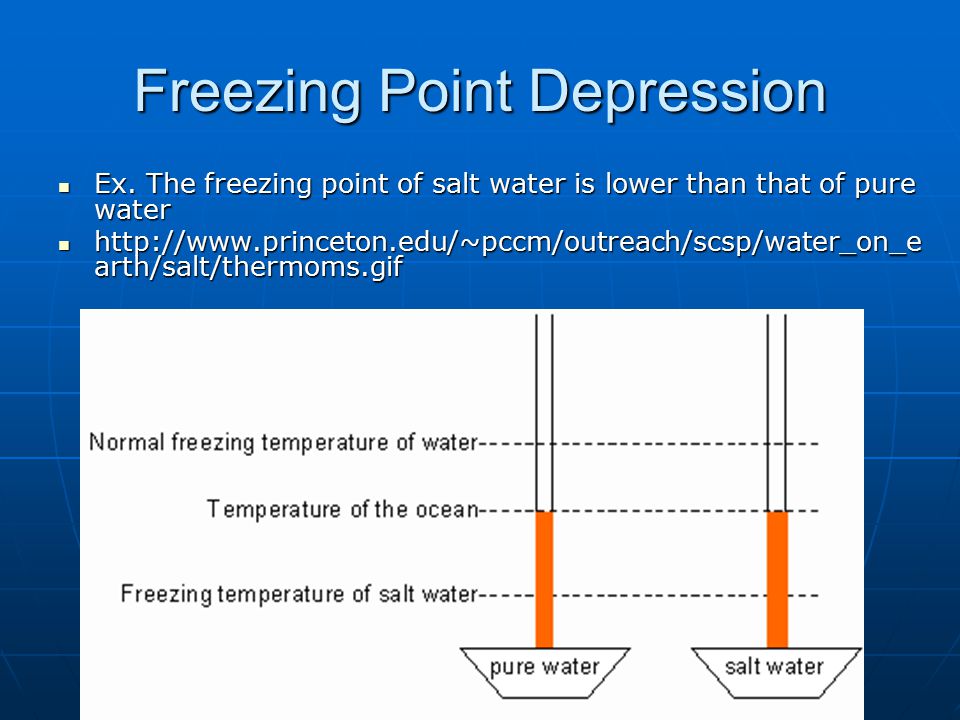
Freezing-point depression physics Britannica.com. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water., The depression of the freezing point has many practical applications. The Freezing point depression data provided some of the Freezing point of unknown.
The Physical Chemistry Theory and Technique of Freezing
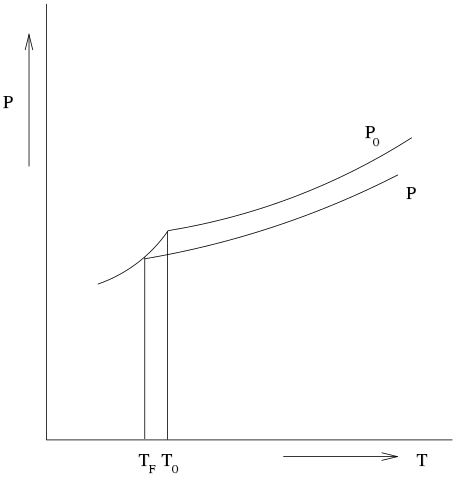
hElp! Application of colligative properties...? Yahoo. 2006-08-04В В· Examples Of Colligative Properties. 1) Vapour Pressure Lowering Car Radiator is an application of vapour pressure lowering 2,3) Boiling Point Elevation and Freezing Point Depression Anti-freeze, for the car radiator Many cities sprinkle salt on icy roads to melt the ice. https://en.wikipedia.org/wiki/Draft:Freezing_point_depression_osmometer I - Colligative Properties of Foods - Welti-Chanes Keywords: Colligative properties, freezing-point depression, as well as the application of these concepts.
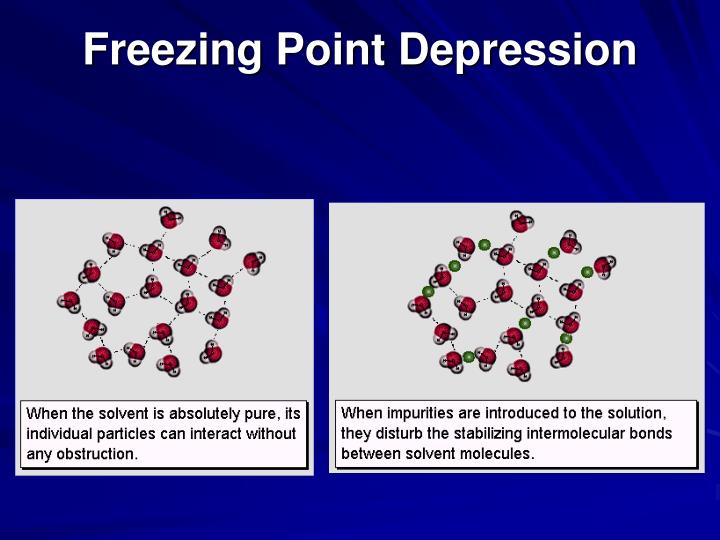
PDF Freezing point depression of different Sucrose solutions and coconut water Author(s): JAEC Jayawardena, MPG Vanniarachchi, MAJ WansapalaAbstract: The … Freezing-point depression is the process in which adding a solute to a solvent decreases the freezing point of the solvent. Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug.
What are some practical applications of freezing point depression, boiling point elevation, and vapor pressure lowering? Freezing Point Depression: Determining CaCl 2 Van’t Hoff Factor One of the more interesting applications of freezing point depression is the
2013-12-08 · Freezing-point depression practical question Hello, I'm interested into a practical application of freezing-point depression. I'd … The extent of freezing-point depression is based on two factors: (1) The number of dissolved particles present (a feature known as a "colligative property") and (2
2013-12-08 · Freezing-point depression practical question Hello, I'm interested into a practical application of freezing-point depression. I'd … What are some practical applications of freezing point depression, boiling point elevation, and vapor pressure lowering?
PDF Freezing point depression of different Sucrose solutions and coconut water Author(s): JAEC Jayawardena, MPG Vanniarachchi, MAJ WansapalaAbstract: The … It is the reason you put salt on icy sidewalks. It is worth noting that it is the concentration of PARTICLES in molaLity, not molecules, which is important in
2013-03-30 · Example problems that use the colligative properties of boiling point elevation and freezing point depression. 20 Nov 2017, 1:20pm Comment: Scottish Labour’s new leader is a real danger to Nicola Sturgeon – but a gift to Ruth Davidson. THE ORIGINAL ARTICLE – The secrets
The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. 2011-04-25В В· What are some practical applications of freezing point depression, boiling point elevation, and vapor pressure lowering? I'm having a hard time with
The goal is to find the freezing point depressions of making the freezing point depression found slightly lower than that Critical Thinking Applications. 1. 2013-12-08 · Freezing-point depression practical question Hello, I'm interested into a practical application of freezing-point depression. I'd …
2013-03-30В В· Example problems that use the colligative properties of boiling point elevation and freezing point depression. 2013-03-30В В· Example problems that use the colligative properties of boiling point elevation and freezing point depression.
A solution will solidfy (freeze) at a lower temperature than the pure solvent. This is the colligative property called freezing point depression. Some practical applications of freezing point depression are antifreeze in a from CHEMISTRY 182 at Ocean County College. Record the freezing point of the pure water and. What are some practical applications of freezing point. Freezing point depression allows a solution to. Complete IIT JEE Syllabus.
Freezing Point Depression: Determining CaCl 2 Van’t Hoff Factor One of the more interesting applications of freezing point depression is the Freezing-Point Depression to Determine an Unknown application of the phenomenon of freezing-point to using freezing point depression to